NEET 2020 Question paper was average in terms of difficulty as reported by the test takers. That year, 1900 applicants attempted the paper in Urdu language.
- The paper had a total of 180 Questions but in 2021, the total number of questions in NEET was raised to 200. Candidates were also given internal choices. Check NEET Exam Pattern
- In total 16.2 Lakh candidates registered for the exam. 7.7 lakh candidates qualified for the exam.
- Top NEET Colleges Cut off in the 2020 session was: AIIMS, Delhi (AIR 51); JIPMER, Puducherry (AIR 90), Maulana Azad Medical College (AIR 89); VMMC, New Delhi (AIR 138).
Aspirants can download NEET 2020 Question paper in Urdu from the links below to get a better idea about the type of questions and difficulty level of the paper.
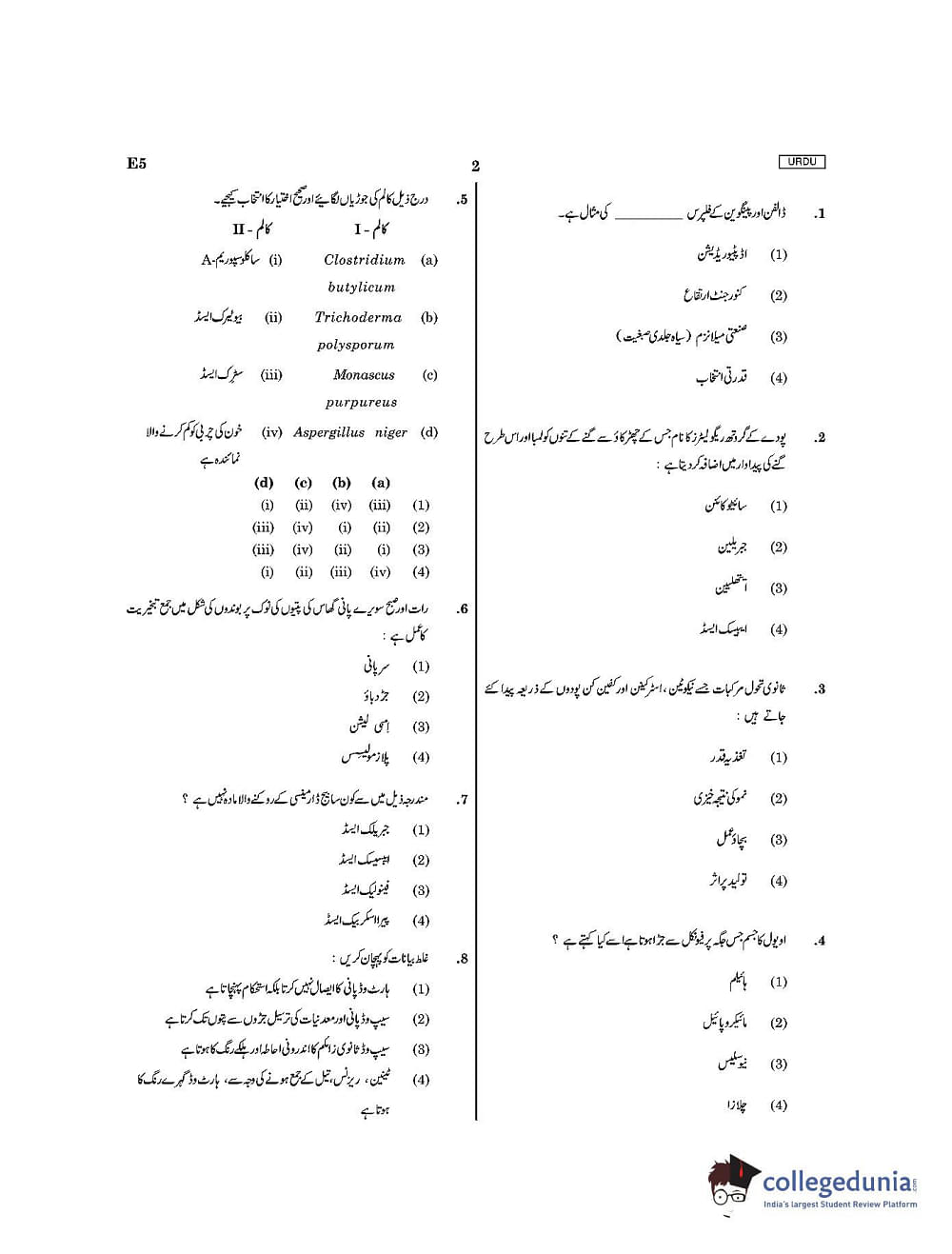
NEET 2020 Question Paper with Answer Key PDF in Urdu for E5 Paper Code
| NEET 2020 Question Paper PDF Urdu E5 | NEET 2020 Answer Key PDF Urdu E5 |
|---|---|
| Download PDF | Download PDF |
























Quick Links:
- How to Prepare for NEET Chemistry?
- How to Prepare for NEET Physics?
- How to Prepare for NEET Biology?
- Download NEET Practice Papers
NEET 2020 Question Paper: Weightage of Class XI and XII Syllabus
The weightage of class XI and XII NEET Syllabus in the 2020 paper was as follows:
Physics
| Parameters | Class XII | Class XI |
|---|---|---|
| Total Number of Questions | 27 (60%) | 18 (40%) |
| Topic-wise distribution |
|
|
Chemistry
| Parameters | Class XII | Class XI |
|---|---|---|
| Total Number of Questions | 24 (53.3%) | 21 (46.7%) |
| Topic-wise distribution |
|
|
Botany
| Parameters | Class XII | Class XI |
|---|---|---|
| Total Number of Questions | 18 (41.9%) | 25 (58.1%) |
| Topic-wise distribution |
|
|
Zoology
| Parameters | Class XII | Class XI |
|---|---|---|
| Total Number of Questions | 22 (46.9%) | 25 (53.1%) |
| Topic-wise distribution |
|
|
NEET Previous Year Question Papers with Answer Key PDFs
NEET aspirants can download NEET previous years’ question papers with answer key PDFs from the links given below for free.
Other UG Entrance Exams
NEET has now replaced JIPMER and AIIMS exams. Candidates wanting to take admissions in Pharmacy, Nursing, Lab Assistant, B.Sc. Biology, and other related medical courses, can check and download the following question paper pdfs.







Comments