Latent heat is defined as the amount of heat required to change the state of a unit mass of a substance without a change in its temperature and pressure.
- Water is found to be in three states: (1) Solid state: Ice (2) Liquid state: Water (3) Gas state: Steam or Vapour.
- The change of material from one state to another is called a phase transition or phase change.
- These involve heat transfer despite no change in the temperature of the substance undergoing the phase.
- The two forms of latent heat are (1) Latent heat of fusion or Melting and (2) Latent heat of vaporization or Boiling.
- For water, the two forms of latent heat are also called: (1) Latent heat of ice and (2) Latent heat of steam
- The heat flow from one object to another may alter either the average kinetic energy of the random motion of the molecules, thus changing the temperature of the object.
- It may otherwise alter the average potential energy of the molecules, which changes the phase of the object.
| Table of Content |
Key Terms: Latent heat, Specific latent heat, Phase transition, Temperature, Energy, Latent heat of fusion, Latent heat of vaporization, Condensation, Freezing, Melting.
Also Read: Specific Heat Capacity
What is Latent Heat?
[Click Here for Sample Questions]
Latent heat refers to the energy released or absorbed by a body of the thermodynamic system, during a constant-temperature process. Alternatively, it may be defined as energy in hidden form, supplied or extracted to alter the state of a substance without changing its temperature.
- Latent heat is associated with the change of phase of atmospheric or ocean water, vaporization, condensation, freezing, or melting.
- For instance, when a body is heated at constant temperature by thermal radiation in a microwave field, it may expand by an amount described by its latent heat with respect to volume or latent heat of expansion, or increase its pressure by an amount described by its latent heat with reference to pressure.
Change of State
Two common types of latent heat are:
- Latent heat of fusion: Latent heat of fusion refers to the heat absorbed or released by a substance during the transition from solid to liquid phase at a constant temperature.
- Latent heat of vaporization: Latent heat of vaporization refers to the heat absorbed or released by a substance during the transition from liquid to gas phase at a constant temperature.
These names describe the direction of energy flow when changing from one phase to another- from solid to liquid, and from liquid to gas.
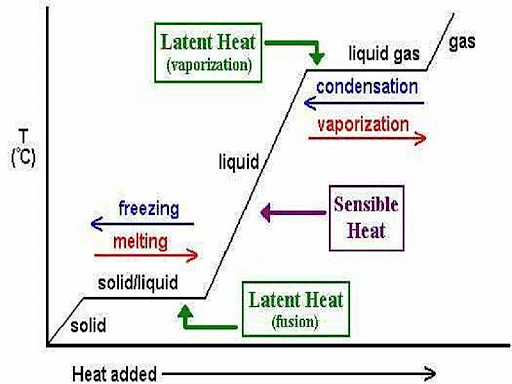
- For example, when a pot of water is heated on a stove, the temperature first starts rising. When it reaches 100°C (212°F), it stops increasing, even though the flame keeps supplying heat at the same rate.
- This heat is utilized in breaking the bonds between the molecules, as the kinetic energy of the molecules remains constant.
- Gradually, all the molecules gain sufficient energy to overcome the intermolecular forces binding the molecules to one another.
Also Read: Heat of Vaporization Formula
Specific Latent Heat
[Click Here for Sample Questions]
Specific latent heat (L) refers to the amount of energy in the form of heat (Q) required to completely affect a phase transition of a unit of mass (m), usually 1 kg, of a substance as an intensive property.
It is calculated using the formula:
L = Qm
Where
- Q = Amount of energy released or absorbed during the change of phase of the substance.
- m = Mass of the substance
- L = Specific latent heat for a particular substance

Temperature v/s Heat supplied graph
Also Read: Class 9 Science Chapter 8 MOTION
Latent Heat of Water
[Click Here for Sample Questions]
The latent heat of water is defined as the amount of heat required to change the state of a unit mass of water without a change in its temperature and pressure. The latent heat of water is of two forms:
- Latent heat of ice: Latent heat of ice refers to the heat absorbed or released by water during the transition from the ice to the liquid phase at a constant temperature. At a pressure of 1 atm, its value is 80 cal/g or 3.33 x 104 J/kg. That means 80 cal of heat energy is required by 1 gram of water to change its phase from ice at 0oC to liquid at 0oC.
- Latent heat of steam: Latent heat of steam refers to the heat absorbed or released by water during the transition from the liquid phase to the gas phase at a constant temperature. At a pressure of 1 atm, its value is 540 cal/g or 22.6 x 105 J/kg. That means 540 cal of heat energy is required by 1 gram of water to change its phase from water at 100oC to vapor at 100oC.
When any material is heated to the temperature where it changes its state (solid to liquid, or liquid to gas), the temperature will remain the same until all the material changes state. These transitions require a larger amount of energy because it takes more energy to convert a substance from one physical state to another.
- When a pot filled with water is placed on the stove, it gradually warms up till the temperature reaches 212° F (or 100° C).
- It will stay constant until the entire water is boiled.
- Similarly, when water freezes, it keeps cooling till the temperature reaches 32°F (or 0° C). It will stay constant until the entire water freezes.
- On heating any substance to the temperature where it changes state, the temperature will stay constant till the entire substance changes its state.
- Thus, ice water will remain at 0° C (32° F) until the entire ice melts, and the same thing applies to all phase transitions.
- This is because the energy must be used to change the state from solid to liquid or liquid to gas.
- Similarly, energy must be withdrawn to change the state from liquid to solid or gas to liquid.

Latent Heat of Water
Also check:
Things to Remember
- The term ‘latent heat’ was introduced around 1762 by British chemist Joseph Black. He used the term in the context of calorimetry where a heat transfer caused a volume change in a body while its temperature was constant.
- Specific heat is recorded in "calories" for “mass in grams” (and “Joules for kg”).
- Conversion of 1 gram of ice at 0° C to 1 gram of water at 0° C requires 80 calories.
- The conversion of 1 gram of water at 100° C to 1 gram of steam at 100° C requires 540 calories.
- Latent heat arises from the work required to beat the forces that hold together atoms or molecules in a material.
Sample Questions
Ques. What is the latent heat of ice? (1 mark)
Ans. The latent heat of ice in calories per gram is 80. In the SI unit, it is 3.36 x 105 J/kg.
Ques. Can a thermometer measure latent heat? (2 marks)
Ans. Usually, a thermometer reads temperature and a calorimeter measures calorific value or heat. Latent heat is the heat necessary for the change of phase. Hence, temperature change during the supply of heat cannot be measured by a thermometer.
Ques. How do you distinguish between latent and real heat? (2 marks)
Ans. The amount of heat needed to increase the temperature of a substance by a certain amount is known as specific heat. On the other hand, latent heat refers to the amount of heat emitted during a change of state, such as the boiling of water or the melting of ice.
Ques. m kg of a substance of specific heat capacity s J/kg °C is heated so that its temperature rises from θ1°C to θ2°C. Write down the expression for the heat Q supplied. (2 marks)
Ans. Heat supplied Q = mass × specific heat capacity × rise in temperature
= m × s × (θ2 – θ1)
Ques. Write two advantages of the high specific latent heat capacity of steam, which is about 226 × 104 J/kg. (3 marks)
Ans. The advantages of high specific latent heat capacity of steam are as follows:
(i) The main advantage of the high specific latent heat capacity of steam is room heating in cold countries. The steam generated within the boiler is passed through pipes in radiators fixed within the building.
(ii) In a thermal power station, steam is used as a medium for converting the chemical energy of coal to electric energy. Every gram of steam supplies a great amount of heat energy of about 2260 joules.
Ques. Discuss how the high specific heat capacity of water helps in the formation of land and sea breezes. (3 marks)
Ans. The specific heat capacity of the land is 5 times less as compared to water. Thus, the air above land becomes hot and light and rises up resulting in a drop in pressure of land mass during daytime. Thus, cool air from the sea starts blowing towards and forming a sea breeze.
During the night, both land and sea radiate heat energy. However, the temperature of land falls faster than seawater, because of the high specific heat capacity of seawater. So, at night the temperature of seawater is more than land. Warm air above the sea rises and cold air from land starts blowing towards the sea leading to the land breeze.
Ques. If the amount of heat needed for a phase change is 300 kcal, calculate the latent heat of a 5 kg material. (3 marks)
Ans. Given parameters are,
Q = 300 k.cal
M = 5 kg
The formula for latent heat is given by,
L = Q / M
L = 300 / 5
L = 60 k.cal/kg
Hence latent heat value is 60 k.cal/kg
Ques. At 20°C, a piece of metal has a density of 60g. When immersed in a steam current at 100°C, 0.5g of the steam condenses thereon. Provided that the latent heat of steam is 540 cal/g, calculate the specific heat of the metal. (3 marks)
Ans. Let c be the specific heat of the metal.
Heat gained by the metal
Q = mcΔt
⇒ Q = 60 x c x (100 - 20)
⇒ Q = 60 x c x 80 cal
The heat released by the steam
Q = m × L
Q = 0.5 × 540 cal
By the principle of mixtures,
Heat given is equal to Heat taken
0.5 × 540 = 60 × c ×80
c = 0.056 cal/g ?C
Hence specific heat value is 0.056 cal/g ?C
Also Read:





Comments